Pituitary Adenoma Surgery
1- More than 99% of Pituitary Adenomas are benign.
2- Pituitary adenoma surgeries can be performed through the nose using endoscopic or microscopic techniques. I perform most of the pituitary adenomas endoscopically through the nose, but if necessary, I operate in a small number of cases by opening the skull with microsurgical technique.
3- The surgery takes approximately 1-5 hours. The fastest pituitary adenoma surgery I performed took 20 minutes.
4- I do not put a tampon in the nose after pituitary adenoma surgery.
5- Pituitary adenoma surgery is life-threatening.
6- If pituitary adenoma has caused vision loss, 98% of the time there is improvement after surgery, 1% of the vision does not change, and 1% of the vision gets worse.
7- If there is a cerebrospinal fluid leak after pituitary adenoma surgery, that is, if water flows from the nose, there is a danger of meningitis. If necessary, lumbar drainage should be installed or repaired surgically.
8- After pituitary adenoma surgery, the patient stays in the hospital for 2 to 7 days.
9- Patients who have pituitary adenoma surgery should be followed by endocrinology.
10-Pituitary adenomasymptoms and why Cushing's disease Andacromegaly You can visit the relevant pages of this site to get information about.
When performing Pituitary Adenoma surgery, how do I pass the nose phase at the beginning of the surgery? When mucosal bleeding in the gradually narrowing nostril blocks the person's path, those who do not have knowledge of anatomy cannot continue the surgery. This difficulty also applies to ENT specialists performing FESS surgery. Where is the sphenoid ostium and how to find it? What does the inside of the sphenoid sinus look like? How is the sella floor recognized? What is the position of the carotid and optic nerve relative to each other? Where is the opticocarotid recess (OCR)? I tried to answer these and similar questions in this video I prepared with animation support. I recommend it to be followed by all neurosurgeons and ENT specialists who will perform endoscopic pituitary surgery. I made the animation myself. While watching this video, which I worked hard to prepare, I hope you like it and subscribe to my YouTube channel.
Pituitary adenoma surgery - for doctors
Picture 11a
pituitary adenoma picture gallery
My Pituitary Adenoma surgeries: You can read the Pituitary Adenoma section below, which I published in the book "Basic Neuroendoscopy" edited by GATA Brain and Nerve Surgery Clinic and where I explain with examples how I perform endoscopic pituitary adenoma surgeries.
ENDOSCOPIC PITUITARY SURGERY
Prof. Dr. Bülent DÜZ
Entrance
Pituitary adenomas are one of the common intracranial tumors. They are basically classified into two groups: hormone-secreting and non-hormone-secreting. Depending on the hormone profile it secretes, pituitary adenomas can cause diseases such as acromegaly, Cushing's disease, hyperprolactinemia, or clinical findings such as visual impairment and headache as a result of pressure on surrounding anatomical structures. Although the primary treatment for prolactinomas is medical treatment, patients who continue to grow despite medical treatment, patients who cannot tolerate medical treatment because they cause nausea and vomiting, patients who voluntarily choose surgical treatment because they do not want to use medical treatment for a long time, and patients in emergencies where acute neurological deficits such as apoplexy occur. In such cases, neurosurgical treatment is performed. The first treatment option for patients without successful medical treatment, such as acromegaly and Cushing's disease, is undoubtedly neurosurgical treatment methods. Conventional radiotherapy has been abandoned in treatment due to its negative effects on the optic nerve and brain. Stereotactic radiosurgery (X-knife, Gammaknife, Cyberknife, etc.) options are preferred in patients who have a residual mass after surgery, in patients who do not accept surgical treatment, or in patients with medical conditions that prevent neurosurgical intervention. . Patients with non-functional pituitary adenoma are evaluated according to the size of the adenoma, its compression on the optic nerve, its invasion into the cavernous sinus, its invasion into the sphenoid sinus, and the presence of intracranial extension; As a result of evaluating variables such as the patient's age and medical condition, Depending on whether the patient wants surgery or not, surgical intervention is performed or the patient's visual field, pituitary MRIs and hormonal profile are monitored at periodic intervals; The patient is directed to neurosurgical intervention or stereotactic radiosurgery options immediately or after further follow-up.
In the second half of the 20th century, microsurgical technique and transsphenoidal approach were adopted as standard surgical treatment in the surgical treatment of pituitary adenomas. Methods of reaching the pituitary gland via the nose have a history of approximately 100 years (3) (16) (28) (31) In this classical technique, which is still applied in many surgical centers today, the nasal septum between the nasal passages is found by first entering under the lip or through the nose, and then by going under the septum mucosa, the sphenoid sinus ostia, the anterior sinus wall and from there the sella turcica and pituitary are reached through the sinus. After a special nasal speculum is placed to keep this passage in the nose open, the operation is performed under a microscope using microsurgical hand tools and a fluoroscopy device.
One of the most important minimally invasive surgical techniques that has increased in recent years is "Endoscopic Endonasal Pituitary Surgery", which is applied to pituitary tumors. Today, some surgeons also use the endoscope as an auxiliary tool to visualize hidden anatomical corners during microscopic transsphenoidal pituitary surgery. Many surgeons perform surgery entirely endoscopically. While pituitary adenomas were operated transcranially or microscopically transsphenoidally in our clinic until 2005 (Figure 1,2,3), after 2005, transsphenoidal surgeries began to be performed only with the help of an endoscope.


Figure 1: Preoperative and postoperative MRI images of a 40-year-old male patient with non-functional pituitary adenoma. It was operated via endoscopic endonasal approach.


Picture 2: Preoperative and postoperative MRI images of a 46-year-old male patient with non-functional pituitary adenoma. It was operated once transcranially and once transsphenoidally, and subtotal resection was performed.


Figure 3: Preoperative and postoperative sagittal MRI images of a 48-year-old female patient with non-functioning pituitary adenoma.
history
The first endoscope was discovered 200 years ago by German physicist Philipp Bozzini (1773-1809), who originally came from an Italian family. This first endoscope, consisting of a candle light, reflective mirror and ocular system housed in a tube, was presented at the Vienna Medical Academy in 1806. (3) (16) (51) A breakthrough was made in the optical system in 1948, when Harold Hopkins discovered the zoom lens system and then Basil Hirschowitz, a gastroenterologist, developed the glass-covered flexible lighting cable (fiberoptic cable system). With the development of image transfer and cold light source by Karl Storz (1911-1996), the clinical use of modern endoscopes in their current sense came true. (3, 36)
The term 'endoscopy' was first used by Antonin Jean Desormeaux (1815-1894), a French urologist. Until the end of the 19th century, the use and development of the endoscope was based mostly on bladder, rectum and pharynx inspection (22)
Endoscope in neurosurgery
When we look at the development of the endoscope in neurosurgery, we see that it parallels the use of endoscopes in different fields of medicine since the beginning of the 20th century (2, 17) (3, 61) (19) (21, 65) Hirschmann, known as the pioneer of paranasal endoscopic surgery, broke new ground by using the cystoscope in the observation of the maxillary sinus in 1901. In 1910, endoscopic thoracoscopy and laparoscopy were performed for the first time with a modified cystoscope (19) (18). In the same years, urologist Victor Darwin Lespinasse (1878-1946) from Chicago used the intraventricular endoscope for the first time in 2 hydrocephalic children and performed choroid plexus coagulation for therapeutic purposes. One of them died in the postoperative period, the other survived for 5 years (24) Although Lespinasse was the first to perform choroid plexus coagulation, Walter Dandy is known as the pioneer of neuroendoscopy. In 1922, he carried out the partially successful initiatives named after him (16). In 1932, he described the excision of the choroid plexus via endoscopic means. In 1923, William Mixter reported the first endoscopic third ventriculostomy (3).
Schloffer (1868–1937), an ENT specialist in Vienna, performed the first successful pituitary adenoma surgery via a nasoethmoidal transsphenoidal approach with lateral rhinotomy in 1907.
In 1910, Oskar Hirsch (1877–1965), an ENT specialist in Vienna, under local anesthesia, published the first endonasal, transseptal, transsphenoidal approach.
Cushing made his first endoscopic pituitary examination using Schloffer's technique in 1909. Then he switched to Hirsch's technique and then developed his own technique under general anesthesia with a sublabial incision instead of local anesthesia. (12)
Mortality was 5.6% in Cushing's 231 patients between 1910 and 1925. He abandoned transsphenoidal surgery, stating that mortality was unacceptable due to CSF fistula, difficulty in bleeding control, and development of cerebral edema.
In 1923, Norman Dott (1897–1973), (He is considered the father of neurosurgery in England.) He went to study with Cushing in Boston, Applying the technique he learned from Cushing, he performed 80 surgeries until 1956. He reported the mortality as 0%.
In 1956, Girard Guiot (1912–1998) visited Dott and learned the transsphenoidal surgical technique and began to practice it in France. He added C-arm fluoroscopy to the technique. He has performed approaches to craniopharyngioma, chordoma and parasellar lesions.
In 1967, Jules Hardy, who came from Montreal, Canada, and studied in France, learned the transsphenoidal surgical technique from Guiot and began to use the microscope in his surgeries. (43)(28).
In the 1970s Apuzzo (42), Bushe and Halves (4) used the endoscope as an auxiliary instrument to the microscope in pituitary adenomas with extrasellar extension. Axel Perneczky, one of the leaders of minimally invasive neurosurgery, also contributed to the widespread use of endoscopes in neurosurgery, especially the use of endoscopes as an auxiliary device in microsurgery. Griffith and Veerapen (25Although they described the transnasal-transsphenoidal route without touching the septum in 1987, this approach has not gained popularity until today. Also in 1994, Cooke and Jones (11th) transnasal-transsphenoidal surgery performed under the microscope; They mentioned its superiority because it can be performed without nasal, septal, dental and sinus complications. In such transnasal cases where an endoscope is not used, a nasal speculum or retractor must be used. The endoscope was first used in transnasal-transsphenoidal pituitary surgery in 3 cases by Jankowski in 1992 (32) Gamea in 1994 (22) stated that in 10 operations in which the sublabial-transseptal-transsphenoidal approach was used, the tumor was dissected better with the use of an endoscope in addition to the microscope. It was Jho who popularized the use of endoscopy in pituitary surgery and had the first series with 50 cases (35) (34). Jankowski (32) recommended partial resection of the middle turbinate to facilitate endoscopic surgery during the operation, Jho (35) argues that in necessary cases, breaking the middle turbinate and lateralizing it is sufficient. From the following years until today, Enrico de Divitiis (5) (15) (6), Cappabianca P(5, 6) Amin Qassam (8) (8, 23, 37-40, 48) and Theodore Schwartz (54, 56) have made significant contributions to the development of fully endoscopic techniques in both pituitary surgery and skull base pathologies. The world's largest series of 800 cases was published by Amin Kassam in 2011. (40) Recently, the pure endoscopic approach for pituitary surgery has become quite widespread and many articles comparing endoscopic surgery and microsurgery methods have been published (7) (8) (9) (13) (36) (41) (44) (45) (52) (53) (63) (64). In addition, in recent years, especially following the development of neuronavigation and intraoperative imaging systems, the endoscopic approach has become increasingly preferred (one) (20) (42) (49). In studies with a large number of cases conducted in the USA, it was found that the average postoperative hospital stay decreased from 6.3 days to 3.3 days with the use of minimally invasive endoscopic technique.
Technical
Transsphenoidal pituitary surgery is performed with 5 main methods.
1- Microscopic sublabial transsphenoidal approach
2- Microscopic transseptal transcephenoidal approach
3- Microscopic endonasal transsphenoidal approach
4- Endoscope assisted microscopic endonasal transsphenoidal approach
5- Endoscopic endonasal transcephenoidal approach
Our experiences with the differences of the above methods were widely published in 2008. Here, it will be explained how endoscopic endonasal pituitary surgery is performed..
ENDOSCOPIC pituitary surgery
A. Preoperative preparation (preparation before surgery)
Informing patients in detail about the surgical technique to be applied makes it easier to reduce postoperative pain and other problems. It should also be noted that this is a legal obligation. All patients scheduled for endoscopic endonasal pituitary surgery must undergo a detailed nasal and sinus examination. Complaints such as headache, feeling of pressure on the face, smell problems, postnasal drip, which are symptoms of sinus infection, and previous surgeries and traumas should be questioned. An endoscopic examination of the nasal passages should be performed to investigate whether there are any problems that may affect the surgery, such as infection, nasal flesh growths, nasal deviations, or polyps. In patients with infection or polyps, these problems must be treated medically or surgically before surgery. In this type of surgery, septal or other nasal anatomical variations may sometimes make endoscopic intervention difficult. (Picture 4). In this case, the wide opposite nasal passage can be used, or turbinate resection or nasal septum surgery can be added to the operation. Additionally, serious intranasal curvatures can be corrected during pituitary surgery. Paranasal sinus computed tomography (CT) should be taken for each patient to know the structure of the nasal passage and sphenoid sinus. In these images, the turbinates, the structure of the sphenoid sinus, the sphenoid sinus aeration, the localization of the intra-sphenoid sinus septa and their relationship with the sella floor should be carefully studied in the preoperative period. (Figure 5 a,b) If possible, the sphenoid sinus ostium is determined. It should be kept in mind that the sphenoid sinus ostium is located in the upper part of the anterior sinus wall with a probability of 52-89.5% (60). This is very important to prevent complications that may develop perioperatively. It should be noted that sometimes the sphenoid sinus septa may be adherent to the carotid bulge. Before surgery, all patients should undergo a complete physical examination, neurological examination, endocrinological evaluation of hormone levels and visual field examination, as well as computerized sinus tomography in a format suitable for navigation and magnetic resonance (MR) examinations for pituitary pathology.

Figure 4: Preoperative septal defect appearance in a case planned to undergo endoscopic pituitary surgery.

Figure 5a: Paranasal CT axial section view of the intra-sphenoid sinus septum

Figure 5b: View of the intra-sphenoid sinus septum during endoscopic surgery. I repositioned the axial section front-back and right-left to match the intraoperative view. Yellow line: intra-sphenoidal septum. Blue triangle: midline.
Positioning:
With the patient in the supine position, the back can be flexed approximately 0-20 degrees. However, flexion or extension of the head should be planned and adjusted according to whether the operating field will be directed from the base of the sella towards the planum sphenoidale or towards the clivus. Without the head deviating to the right or left It is fixed in the midline with a 3-spike head stabilizer. Since the surgeon will work on the patient's right, the head may be deviated to the right within the range of 0-20 degrees, depending on experience. However, we recommend not to deviate the head to avoid losing the midline. (Picture 6)


Picture 7: After entering the left nasal cavity, the middle turbinate is deviated laterally with the help of an aspirator and a path is opened towards the sphenoid osteas.
The nasopharynx and choana are identified and the choana is approximately 1-1.5 cm away. Above it is the superior turbinate, and the sphenoid ostium is reached inferomedially of the superior turbinate. The sphenoid sinus ostium is a marker for the anterior wall of the sphenoid and the floor of the sella. Sometimes it may be difficult to determine the location of the ostium. It may be useful to use the navigation system for this. It should not be forgotten that the superior nasal meatus and superior turbinate are important in the localization of the ostium (3, 60). The approach through the superior meatus facilitates the localization of the ostium and provides less damage to the middle turbinate. In addition, preservation of the middle turbinate allows it to be used as a graft at the end of surgery to prevent cerebrospinal fluid (CSF) fistula during closure (17) (59). After the sphenoid ostium is located, the surrounding area is cauterized.

Figure 8: After the tissues on the anterior wall of the sphenoid sinus are coagulated with the aspirator tip, the vomer, the anterior wall of the sphenoid sinus and both sphenoid osteums are seen on top. The borders of the anterior wall of the sphenoid sinus are seen with white stripes, and the sphenoid osteum is seen in green.
Sphenoidal stage:
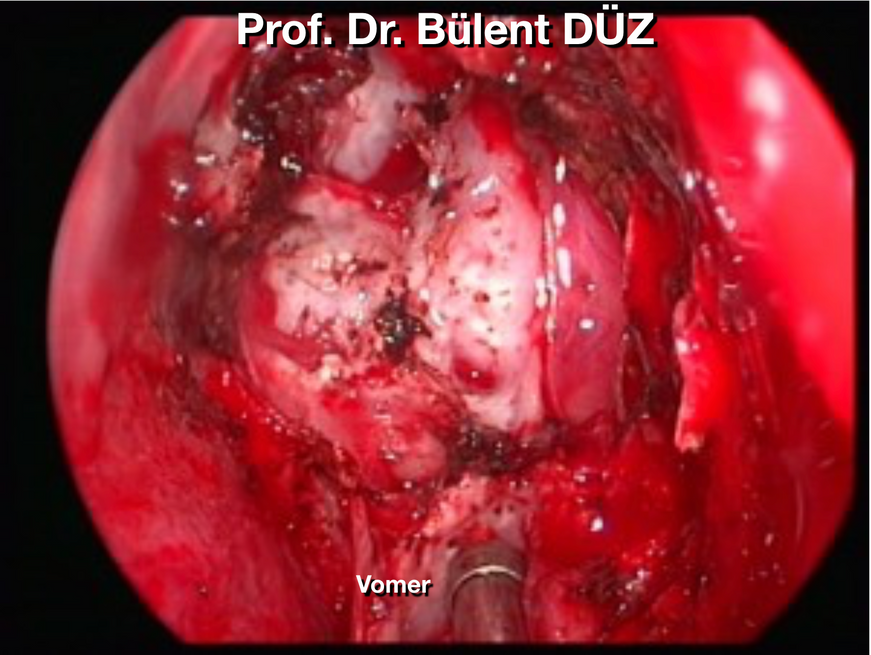
The mucosa around the sphenoid sinus ostium and the posterior parts of the nasal septum is cauterized and scraped. Then, the posterior edge of the septum is separated from the crista sphenoidalis and passed to the opposite side to find the opposite sphenoid ostium. (Figure 7, 8). The posterior part of the septum and the rostral protrusion of the sphenoid sinus are burned from top to bottom with a cautery approximately 1.5-2 cm long, and the nasal septum cartilage is stripped from the vomer and sphenoid rostrum and lateralized. What should be noted here is that the posterior lateral septal artery originates from the sphenopalatine artery and passes through the posteromedial corner of the inferior border of the middle turbinate, feeding the nasal septum. The posterior lateral septal artery should be well controlled to avoid both intraoperative and postoperative bleeding. This procedure is easily performed with an insulated cautery with aspirator feature. Another important point in cauterizing this area is that if the middle turbinate is planned to collapse into the sphenoid sinus in case of CSF leakage at the end of the surgery, the posterior inferolateral connection of the turbinate should not be burned while cauterizing it. If the posterior inferolateral connection of the middle turbinate is preserved, the turbinate can be cut from anterior to posterior with nasal scissors and tipped with a pedicle towards the posterior, that is, into the spheonoid sinus, using its soft preserved connection. The nasal septum is 1.5-2 cm from the sphenoid rostrum and the vomer. After it is separated from top to bottom and lateralized, 1 cm of the posterior part of the nasal septum separated from the sphenoid bone can be resected. The mucosa around the opposite side ostium is also cauterized and dissected, thus a larger working cavity can be obtained. Both ostia and the anterior wall of the sphenoid sinus are completely exposed. During dissection and removal of the mucosa, special devices that break the mucosa into small pieces and aspirate them can be used. Using a Kerrison rongeur, high-speed drill or similar instruments, an anterior sphenoidotomy is performed, starting from the ostium on the studied side and moving towards the ostium on the opposite side. During this procedure, it should not be forgotten that the sphenopalatine artery is inferolateral and should be protected (60) . To prevent damage to vital structures, the lateral part of the ostium and the inferior wall forming the sinus floor should not be removed for sinus obliteration during the closure process. The sphenoid sinus septa are not always in the midline, so knowing the septa anatomy in advance is important to avoid losing the midline plane. (Figures 5 and 9) Since it is used transnasally, it should be taken into consideration that the endoscopes are not parallel to the midline. In some cases, posterior septectomy may also be required. However, the wider the anterior sphenoidotomy is made, the easier it is to manipulate the instruments at the sellar stage (Figures 5b, 8, 9).

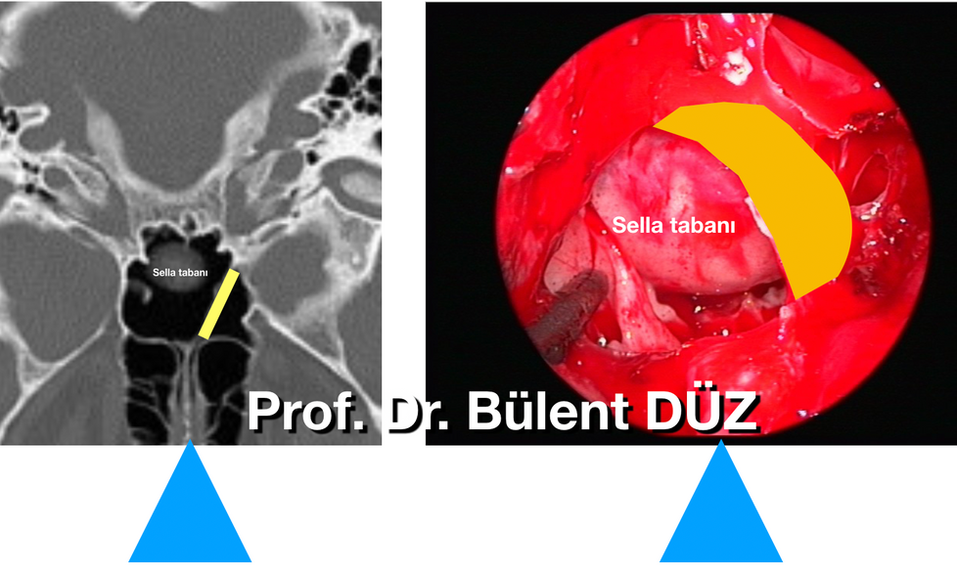
Figure 9: After the anterior sphenoidotomy is performed, the sella floor is seen inside. The tip of the aspirator points to the posterior wall of the clivus under the sella. There is carotid protuberance on both sides. To the left of the aspirator, the intrasinus septum, which extends across the sphenoid sinus, is seen to adhere to the sella floor and clivus posteriorly in the posterior. Carotid protuberance is seen on both sides.
The septa within the sphenoid sinus must be resected to leave room to easily reach the sella floor. Sometimes the septa come from the lateral side and their base adheres widely to the carotid protuberance. These need to be reduced carefully with the kerrisonr rongeur because they can cause carotid injury if their bases are fractured. There is no need to completely remove the septs within the sphenoid sinus. It is sufficient to expose the entire sellar floor. The septa within the sphenoid sinus should be evaluated as anatomical guide structures by thoroughly examining the coronal and axial paranasal sinus tomographies taken before the surgery. After the anterior sphenoid wall is removed, both optic and carotid ridges, both opticocarotid recesses, and the base of the sella should be identified. (Picture 10) The sphenoid mucosa was burned from the middle and It should be protected by pushing it to both sides. In this way, the normal mucosal lining can recover after sella floor reconstruction at the end of the surgery. There are publications recommending that the sphenoid sinus mucosa be preserved as much as possible and that the tumor spreads towards the base of the sella after being evacuated. (33) It may be difficult to determine the sella floor within the sphenoid sinus when there are anatomical variations or due to the mass effect of the tumor or distortion of facial anatomy in acromegaly. The navigation device can also be used to confirm the anatomical region (Figure 10,11 a, b,c). The navigation system gains greater importance, especially in cases where anatomical markers are lost due to tumor expansion or in cases that will be reoperated (61) .
Before proceeding to the sellar phase, both nostrils must be prepared as described above, so that the endoscope can be advanced into the sphenoid sinus from both nostrils, in order to ensure binostril study. First, we use the left nostril to find both ostia of the sphenoid sinus and remove the front face of the sphenoid sinus with Kerrison rongeurs. Once there is sufficient working space inside the sphenoid sinus, we enter the other nostril and lateralize the other middle turbinate to provide easy access to the inside of the sphenoid sinus. At this stage, since the posterior edge of the nasal septum contaminates the tip of the endoscope, bite back 0.5 cm from the posterio of the nasal septum to provide a wider working and illumination area. We perform resection with rongeurs (backbiter).
Sellar stage: The first step in this stage is to first open the sella floor in a small way and then widen the defect with the help of kerrison reongeurs. (Picture 10). Since this area is sometimes weakened by the tumor, it may be easy to reach the target in giant adenomas.

Figure 10: Wide resection of the sella floor.


Picture 11b
Picture 11a, b: Sella floor may be anatomically different in some cases. In this case with a different sella base, bone resection was performed after determining its location by navigation.

Picture 11c: With the help of intraoperative CT-navigation, the anterior wall of the sphenoid sinus is first reached (upper images), then it progresses within the sinus and the sella floor is reached and confirmed by navigation (lower images).
In cases where there is no bone destruction, it may be necessary to use a high-speed drill or hammer-chisel. By using the visual advantage of the endoscope, the sella floor dura can be opened wider and the sella floor opening can be widened with the help of Kerrison rongeur to evacuate more tumor and to have a better understanding of the anatomy, up to the limits of both carotid bulges. (Figure 11,12). Opening the sella floor wide is especially useful in giant adenomas. However, after determining the borders of the adenoma with the help of navigation in microadenomas within the sella that are clearly evident in preoperative MRIs on the right or left side, We find it sufficient to create a defect at the base of the sella just enough to remove the adenoma. If the opening we created at the base of the sella during the surgery is not sufficient to remove the adenoma, we can use a more minimally invasive method to widen the defect as needed. We consider it as an approach.

Figure 12: After the sella floor is removed with Kerrison, the tumor capsule inside protrudes outwards.
There are publications that recommend performing a dural puncture to determine the location of the intercavernous sinuses before the dural incision. The dura is coagulated with bipolar as necessary. After the dura is cauterized with bipolar, the incision can be widened upward and downward to provide as wide a working area as possible. The tumor is evacuated using endoscopic hand tools using a known microsurgical technique (5) (Picture 13) Dr. in the removal of pituitary adenomas. The method of tumor massage with double aspirators, defined by Amin Kassam, is the method of stroking the tumor with aspirators designed in various widths and inclinations, emptying it, then finding the border of the tumor in the arachnoid plane and then dissecting it. According to this method, a double aspirator is also used to remove the adenoma. However, this method is extremely time consuming. The method we use is to first biopsy the adenoma whenever possible after opening the dura. Adenomas with a soft consistency leave themselves as soon as the sella dura opens with brain pulsation. For this reason, it is necessary to warn the surgical team when opening the sella floor and to keep the necessary pituitary rongeur and biopsy container ready for rapid biopsy. Otherwise, the adenoma that drains into the sphenoid sinus with blood may go to the aspirator and the required amount of pieces for pathology may not be taken. Almost half of the pituitary adenomas are not very soft in consistency. . In this case, we first drain the mass from the middle part using a ring curette and remove the emptied parts with a pituitary rongeur to provide pathological sampling. After taking a sample for pathology, we try to evacuate the edges of the tumor as much as possible with the help of an aspirator. If the tumor comes to the aspirator easily, it is easier for the adenoma to drain. However, if it is not easy to use the aspirator, then the assistant neurosurgeon guides by holding the endoscope, and the neurosurgeon performing the operation takes the aspirator in his left hand and alternately takes the ring curette and pituitary instrument in his right hand, scraping the adenoma from the edges and removing it with the pituitary device. Meanwhile, there is an endoscope above and an aspirator below in the right nostril, and a ring curette or pituitary device in the left nostril. Since the neurosurgeon's three-dimensional perception adapts to the depth of the aspirator held by the left hand during the surgery, the neurosurgeon's right hand can operate instruments such as Rigg curette, pituitary rongeur, or kerrison rongeur in the patient's left nostril easily and without damaging the surroundings. (Picture 14) Waterjet dissection or ultrasonic aspirator can also be used to evacuate hard tumors (46) (47). In order to avoid damaging the lateral ICAs in the removal of giant adenomas, Doppler ultrasonography can be used before or after the dura is opened while removing the tumor. (Picture 15)

Picture 13: Removal of adenoma using aspirator technique with aspirator and ring curettes

Figure 14: Use of the endoscope and handpiece by a single neurosurgeon during the nasal phase of the surgery. There is an endoscope in the left hand and an aspirator and cautery in the right hand. The patient's left nostril is studied.

Figure 15: Determining the position of the ICA laterally while evacuating the tumor after opening the dura during surgery using Doppler ultrasonography.
The residual supra and parasellar areas are checked with 30-45° angled endoscopes and the residues are evacuated under the guidance of the endoscope. If available, these areas can be checked using navigation, intraoperative CT or MRI device. In this way, it can be determined whether there is residual tumor in the suspected areas.
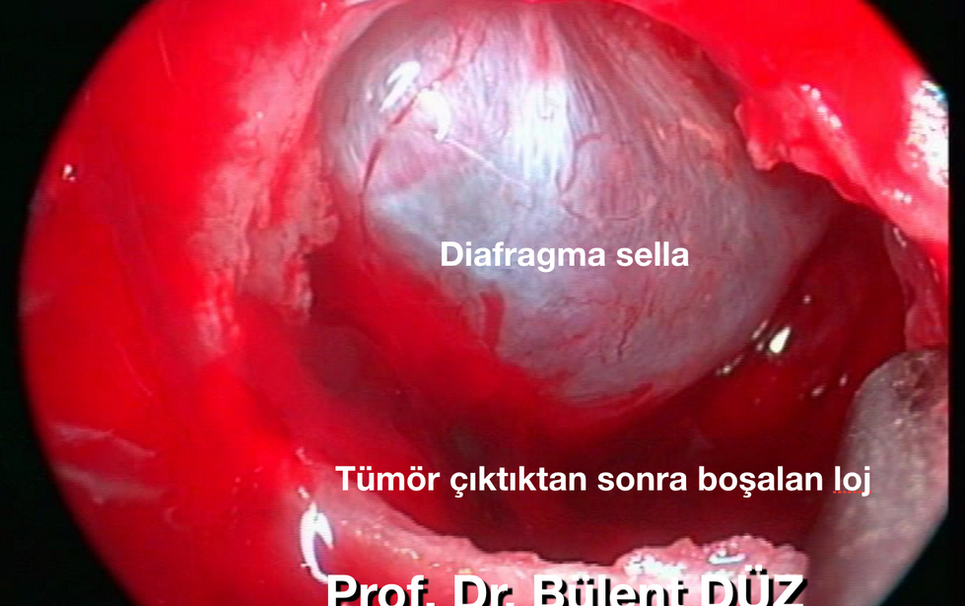
When examined carefully before surgery, it can be seen to which side the adenoma lateralizes the pituitary. (Picture 16) It is possible to distinguish pituitary adenoma from tumor tissue during surgery in almost all cases. The pituitary itself expands at the end of the surgery as a red and highly vascular structure. (Figure 16, 17a,b,c). However, in some cases of giant adenoma, the pituitary tissue may have become extremely stretched and thinned and spread over the diaphragm sella as an arachnoid-like membrane. When normal pituitary tissue is seen, it should be preserved as much as possible in nonfunctional adenomas. If the pituitary itself is attempted to be mobilized with a curette or aspirator after removal of the adenoma, postoperative temporary diabetes insipidus is highly likely. However, diabetes insipidus is detected by hourly urine monitoring and controlled by administering a single dose of half or 1 ampoule of Minirin. In hormone-active adenomas that have caused Cushing's disease or acromegaly, we resect the pituitary adjacent and adherent parts of the adenoma by burning with bipolar to eliminate microscopic invasions.
The Valsalva maneuver is then performed to ensure there is no bleeding or CSF leak. In large adenomas, when the tumor is completely removed from the middle and above, the diaphragm sella falls further and protrudes into the sphenoid sinus. (Figure 18) In this case, residual tumor remains in the lateral carotid grooves. For this reason, strategically, in large adenomas, the adenoma should first be evacuated from the inferior at the base of the sella, then from the right and left lateral carotid grooves, and then directed upwards, and the parts of the adenoma that are adjacent to the diaphragmatic sella should be removed with a ring curette or aspirator. After the diaphragm sella collapses and protrudes, it is extremely difficult to resect the remaining adenoma by pushing cotton pads or strips inside. If there is no CSF leak, struggling with the diaphragm sella usually causes perforations in the diaphragm sella and CSF leakage. In this case, if the adenoma is nonfunctional and 90% of the adenoma has been removed, that is, if sufficient decompression has been performed for the safety of the optic pathways, closure can be started. (Picture 20, 21, 22, 27, 28)

Picture 16: Sagittal and coronal MRI images of GH-secreting pituitary adenoma in a patient with acromegaly. The pituitary itself is seen to be displaced posterosuperiorally within the sella, retaining intense contrast in contrast-enhanced MRI images. Endoscopic endonasal excision of the tumor, the tumor protrudes into the sphenoid sinus and is seen as dark red at the tip of the aspirator.

Figure 17 a: Endoscopic removal of the pituitary adenoma that has destroyed the sella and advanced towards the sphenoid sinus, guided by navigation. Visualization of tumor tissue within the sphenoid sinus in the intraoperative image. Tumor tissue was confirmed by navigation.

Figure 17b: After the tumor tissue extending to the sphenoid sinus is removed, the sella is entered and the pituitary adenoma is evacuated. Tumor tissue within the sella is also confirmed by navigation.

Figure 17c: Step-by-step removal of the tumor is seen with the help of navigation (left column) and intraoperative MRI (right column) system.

Figure 18: While the giant adenoma was being removed, the diaphragm sella protruded. A thinned pituitary gland can be seen attached to the diaphragm sella. An attempt is made to remove the adenoma pieces located in the intrasellar carotid groove between the diaphragm sella and the carotid using a curved aspirator.

Figure 19a, b: Preoperative contrast-enhanced sagittal and coronal MRI images of macroadenoma. Figure 19c,d: Contrast-enhanced sagittal and coronal MRI sections of the same patient in the 3rd postoperative month. Figure 19 e: After the adenoma was completely removed, the diaphragm sella protruded into the sphenoid sinus. I showed the protruded diaphragm sella on the side with a drawing like a football.

Figure 20: Total removal of pituitary macroadenoma step by step with the help of intraoperative MRI system.

Figure 21: Preoperative and postoperative day 1 MRI images of non-functional pituitary macro adenoma

Figure 22: Preoperative and postoperative MRI images of non-functional pituitary macro adenoma

Picture 23/1: Seeing the axial, sagittal and coronal images of the adenoma on the navigation screen in pituitary adenoma surgery.

Picture 23/2: Different stages of adenoma removal seen on the navigation screen are shown with photographs.

Resim 23/3:Bu ameliyatta kapatmada orta konkanın devrilmesinden sonra sfenoid sinüs içi, devrilmiş orta konka ve koana gösterilmiştir.
KAPATMA-SELLA TABANI REKONSTRUKSİYONU
Diafragma sella görülüp, BOS kaçağı izleniyorsa sella içine kaçağın olduğu bölgeye yağ dokusu yerleştirilir ve kaçak tıkanır (Resim 24,25,26). Sella içindeki kanamaların da kontrol edilebilmesi için yağ dokusunun üzerine ince bir tabaka surgicel sarılarak sella içine yerleştirilmesi oldukça faydalı olmaktadır. Sfenoid sinus içerisine ufak spongostan parçaları konulabilir. Vaskülarize nazoseptal flep veya orta konka da kapamada ve BOS fistülünü engellemede kullanılabilir (10) (17) (30) (59).
Hipofiz adenomunun çıkarılması bitince kapatma aşamasına geçilir. Kapatma ve sella tabanı tamiri adenomun mikroadenom, makroedenom veya dev adenom olmasına göre ve ameliyat esnasında BOS kaçağı olup olmadığına, diafragma sellanın delinerek BOS kaçağı olması, diafragma sellada geniş bir defekt nedeniyle BOS kaçağı olması veya 3. Ventrikülün tabasınının açılması nedeniyle BOS kaçağı olmasına göre farklı metodlarla yapılmalıdır. Kapatmada ortak nokta tabaka tabaka kapatma işleminin planlanmasıdır.
-
Tabaka:Kapatmada önce sella içindeki kavitenin büyüklüğüne göre yağ konması postoperatif BOS kaçağı riskini azaltmaktadır. Yağa surgicel sarılarak veya sarılmadan sella içine konması aynı zamanda sella içinde küçük sızıntı halindeki kanamaları kontrol etmekte son derece faydalıdır. Mikroadenomlarda sella içine yağ konmayabilir. Bazen diafragma sella sella tabanındaki defekten dışarı doğru basınçla protrude olmaktadır. Bu durumda da içeriye yağ koymak mümkün olmamaktadır. Dev fibrotik adenomların rezeksiyonundan sonra oluşan çok geniş volümün tamamının yağ ile doldurulması istenmiyorsa yağ dokusunun yanısıra sella içine doku yapıştırıcısı da sıkılarak sella içinde geniş ölü boşluk oluşadak BOS kaçağı olması engellenebilir.
-
Tabaka: Epidural dura tamiri (inlay layer): Sella içindeki dura ile sella arasına dura yerleştirilmesi. (Resim 23/2k, 24) Dura ya ya dura yerine geçecek allogreft ya da laterl femoral bölgeden alınan fascia lata ya da abdomen ön duvarından alınacak fascia olabilir. Biz olguların BOS sızıntısı olduysa abdomen ön duvarından fascia alarak kullanmayı BOS sızıntısı olmadıysa ya da geniş bir diafragma defekti yoksa insan dura allogrefti kullnamayı tercih ediyoruz.
-
Tabaka: sella tabanındaki kemik defekti tamir etmek için ameliyatın başında alınarak saklanan bir parça kemiğin sella tabanına yerleştirilmesi. (Resim 23/2 l, 25) Kemik rekonstrüksiyonu yapılması zorunlu olan bir aşama değildir. Biz olguların üçte birinde kemik rekonstrüksiyonu yaptık.
-
Sfenoid sinüs içi mukozasının tamiri: (Onlay layer-Sellanın sfeoid sinüse bakan yüzünün desteklenmesi) Bu amaçla epidural dura tamirinde olduğu gibi ya insan dura allogrefti ya da fascia kullanıyoruz. Durayı veya fasciayı sfenoid sinüsün içine sellanın tabanına yayıyoruz. (Resim 25)
-
Greftin yapıştırılması: Sfenoid sinüs içine serilen duranın kayarak greft migrasyonu olmasını önlemek için ya bir yüzü yapışkan olan kollejen materyal (Tachocomb) ya da çok az miktarda doku yapıştırıcısı kullanılabilir. Biz doku yapıştırıcısının bu aşamada kullanılmasının eğer introperatif BOS sızıntısı varsa sfenoid sinüs içinde yapıştırma fonksiyonu yerine ölü boşluk oluşturarak dokuların teması ile granülasyon dokusunun gelişimin engelleyeceği düşüncesiyle uygun olmadığı kanaatindeyiz. Ancak sadece geçici olarak tutturmak için çok ince bir tabaka halinde doku yapıştırıcısı kullanılabilir.
-
Pediküllü Orta konka devrilmesi: Geniş diafragma sella defekti varsa veya daha önce transkranial hipofiz cerrhisi yapıldıysa yüksek akımlı BOS kaçağı olma riski yüksek olduğundan sella tabanına orta konka devrilir, yoksa bu aşama uygulanmaz. (Resim 23/1,2,3) Biz genellikle sol orta konkayı kullanıyoruz. Ameliyatın başında lateralize edilen orta konka anteriordan posteriora doğru superiordaki yapışma yerlerinden aspiratör koterle yakılarak posteroinferomedialde bir pedikül kalacak şekilde serbestleştirilir. Orta konka kısmen oftalmik arterin etmoid dalından beslenir, ancak esas beslenmesini maksillar arterin pterigopalatin seğmentinin sfenopalatin dalından almaktadır. Orta konkanın sfenoid ostiumun lateralinde nazal kaviteye tutunduğu yumuşak kısmın yakılmaması yeterli pedikül beslenmesine izin vermektedir. Posteriorda pedikülü sağlam bırakılarak üst kenarı serbestleştirilen orta konka uzunlamasına sella içine devrilerek sella tabanına dışarıdan destek olacak şekilde yaslanır.
-
Pediküllü septal flep çevrilmesi: (26) Eğer çok geniş dafragma sella defekti yoksa ve 3 ventrikül tabanı açılmadıysa yani yüksek akımlı BOS sızıntısı olasılığı yoksa bu aşama uygulanmaz. (Resim 28 a,b,c,d,e)Nazoseptal arter posterior septal arterin bir dalıdır ve nazal septumun her iki yanındaki septal mukoza kendi tarafından gelen bu arterle beslemektedir. Nazal septal mukoza nazoseptal arter korunacak şekilde posteriordan anteriora inferiora ve tekra posteriora doğru aspiratör kotere aracılığıyla yakılarak nazal septumdan sıyrılır. Kapatılmak istenen defektin büyüklüğüne göre nasal septal mukoza inferolateralde pedikülü sağlam bırakılacak şekilde hazılanır ve defekt alanına serilir. Nazal septumun üzeri çıplak bırakılabilir. Nazal septumun üzeri daha sonra ince bir granulasyon tabakası ile kapanmaktadır.
-
Sfenoid sinüsün içine yağ doldurulması: Dışarıdan serilen ve sıyrılmaması için geçici olarak yapıştırılan dura tabakasının içerden sızması olası BOS ile migre olmasını engellemek ve yapılan rekonstrüksiyona granülasyon gelişinceye kadar alttan destek olması için sfenoid sinüsün içini abdominal bölgeden alınan yağ ile oblitere ediyoruz. (Resim 25) Bu yağ dokusu 3-6 ay içinde eriyerek sfenoid sinüsün içi boşalmaktadır.
-
Doku yapıştırıcısı: Yağla oblitere edilen sfenoid sinüsün ön duvarının doku yapıştırıcısı ile kaplanmasıdır. (Resim 26) Doku yapıştırıcısı sıkılırken acele etmemeli yavaşça sıkılmalı ve yapıştırıcının koanaya akmadığından emin olmak gerekir. Yapıştırıcı sıkıldıktan sonra koananın ve nasal hava pasajının açık olduğunu kontrol etmek gerekir.
-
Nasal aşama: Burun içine tampon koymuyoruz. Laterale devie edilen nazal septum karşı burun deliğinden koana kontrol edilirken aspiratörün kenadı ile itilerek tekrar orta hatta konumlandırılır. Kanama ve koananın hava pasajı açısından açık olup olmadığını kontrol ettikten sonra ameliyata son verilir. Gerekirse nelaton sonda şişirilerek de önden bariyer oluşturulabilir. Biz burun içerisinde balon şişirerek destek yapılması veya her iki burun deliğinin merosel ile tampone edilmesi metodlarını artık kullanmıyoruz.
-
Lomber kontunyu drenaj takılması: Eğer geniş diafragma sella defekti nedeniyle introperatif BOS gelişi olduysa ve/veya 3. Ventrikül tabanı açıldıysa, veya hastaya daha önce transkranial operasyon yapılmışsa BOS kaçağını engellemek için hasta 3 ya da 5 gün süresince lomber drenaja alınır.
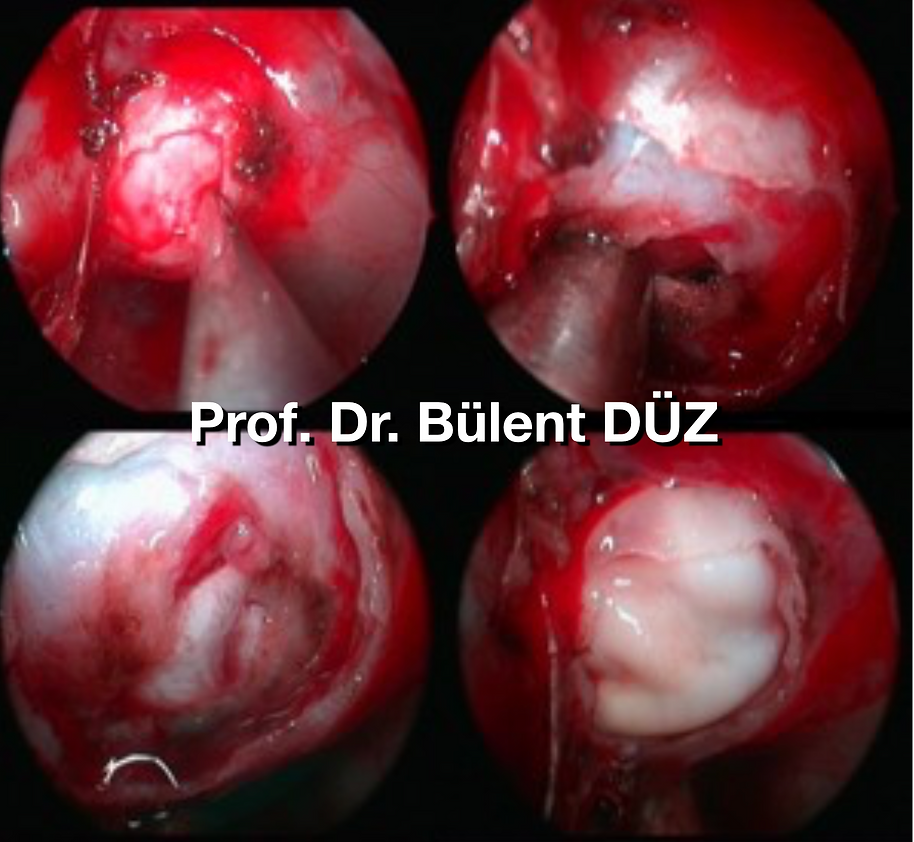
Figure 24: Opening of the sella floor and excision of the tumor (upper images), closure of the sella floor with fascia graft (lower images).

Figure 25: Repair of the sella floor with fascia, fatty tissue and tissue adhesive (Duraseal®)

Picture 26: After the tumor is removed, the sella base is closed and tissue adhesive (Duraseal®) is applied on it.

Picture 27: The patient, who had previously undergone transcranial pituitary adenoma excision, underwent endoscopic transnasal tumor excision under navigation guidance.

Figure 28 a,b,c,d: The part of the reoperated pituitary adenoma case extending to the third ventricle is evacuated with the help of an endoscope. A, Adenoma filling the 3rd ventricle is seen. B shows the use of bipolar cautery when necessary during removal of the adenoma. C, view of the lodge from inside the sella after removing the mass in the third ventricle. D, Following the excision of the adenoma filling the third ventricle, the ventricle walls, foramen of Monro and choroid plexus are seen closely.
Figure 28e: Since the third ventricle was opened after the subtotal removal of the giant and invasive adenoma, the pedicled septal flap technique was used for closure.
Advantages of Endoscopic Surgery
The most important advantage of the endoscope; It is a wide and angled, panoramic field of view provided by active illumination provided by a light source directly approaching the pathology, a fiberoptic-lens system and cameras with improved, highly sensitive image sensor features (15 times more sensitive to light than standard photographic film). In this way, the most important guiding anatomical structures, especially in the transsphenoidal surgical corridor;
• Carotid swelling
• Optical bulge
• The opticocarotid junction can be clearly visualized.
In this way, the midline structures are not deviated from, the sella base can be fully opened, and the radioactive effects of C-arm fluoroscopy, which is used to ensure the midline in microscopic surgery, are avoided. In addition, during and after tumor decompression, all walls within the sella can be viewed and residual tumor tissues can be seen and cleaned by using the angled endoscope. When there is a peroperative CSF leak, the leak site can be seen with an endoscope and a safer occlusion can be made. Since normal anatomical openings are generally used, reoperations can be performed very easily during endoscopic intervention. Additionally, with the angled endoscope, normal pituitary tissue can be observed leaning against the wall after the tumor is emptied in the sella. There is no sublabial or nasal incision in this technique. Therefore, dental, gingival or sinonasal complications are minimal (29) (30) (41) Additionally, since the septal mucosa is not separated, no tampon is placed, so patients breathe more comfortably and easily in the postoperative period. In this approach, if there are no complications, patients can be discharged on the first postoperative day, provided that hormone checks are performed on an outpatient basis.
By protecting the nasal septum and mucosa in endoscopic transsphenoidal intervention;
• Facial and headache, seen in 35% due to nasal packing,
• Atrophic rhinitis,
• Septum perforation,
• Hyposmia, anosmia,
• Alveolar sensory disorder,
• Complications such as deformity in the bridge of the nose are avoided.
Disadvantages
Since only two-dimensional images can be provided with endoscopes, those who are not accustomed to endoscopic surgery may encounter difficulties. The three-dimensional binocular image quality, including the depth used in the use of a microscope, is not available in the endoscope. Since it is two-dimensional and viewed on a monitor, an image with relatively less sharpness and clarity is obtained. In addition, the body of the endoscope narrows the surgical corridor, providing less maneuvering space, and fewer surgical instruments can be inserted into the area. While working with the endoscope, the surgeon has to use only one hand during the operation since he holds the endoscope with one hand. In standard surgery, it is possible for the surgeon to use both hands under the microscope. Jho states that it is possible for the surgeon to use both hands with auxiliary devices that can keep the endoscope stable. In the beginning, the operation time is longer compared to conventional surgery during the adaptation period in the first cases, but this time becomes much shorter as experience is gained.
In the reoperation of previously operated pituitary adenomas, surgery is risky and orientation is difficult because the anatomical structures and markers are disrupted. In this case, navigation systems must be used. The navigation system guides the surgeon, providing wider and more radical excision and preserving important neural and vascular structures (Figure 11c, 17a, b, c, 20, ). When performing endoscopic resection, especially in giant pituitary adenomas extending to the third ventricle, a navigation system must be used and surgical orientation must be provided at every stage (Figure 23,27,29). In addition, since the risk of postoperative rhinorrhea is high in such cases where the ventricle is opened, the closure procedure should be performed very carefully and postoperative lumbar drainage should be applied to the patient.
Complications of Endoscopic Surgery
-
Complications related to the surgical approach:
-
Nasal: Bleeding may occur from the mucosal branches of the sphenopalatine artery. Hyposmia or anosmia may occur (may develop due to excessive coagulation of the upper nasal septum mocosa). If a speculum is used, nasal septum perforation may occur.
-
Sphenoid sinus: Sinusitis and mucocele may occur. Attention should be paid to the carotid bulge and it should be avoided when working in the sphenoid. Bleeding or pseudoaneurysm may develop due to carotid artery injury.
-
Supra and parasellar: Subarachnoid hemorrhage, vascular injury, hematoma, visual impairment may occur. The most common causes of bleeding and hematomas are injuries to the anterior and posterior cerebral arteries or anterior communicating arteries. Sometimes pseudoaneurysm develops due to these injuries. In addition, infarction in the area fed by the injured artery may also be seen in the late period. First of all, bleeding should be stopped with simple methods such as coagulation and surgicel. If this is not successful and a serious hematoma occurs, an emergency craniotomy should be performed on the patient, the hematoma should be evacuated and the bleeding should be stopped. After the surgery, angiography should be performed to find out the cause of bleeding and to reveal other vascular pathologies, if any. If necessary, treatment should be applied with endovascular methods. The cause of visual impairment is optic nerve injury. To avoid this, particular attention should be paid to optic bulge.
-
Endocrine complications:
-
Anterior pituitary failure: Hypopituitarism may be seen.
-
Posterior pituitary failure: Diabetes insipidus is the most common complication.
-
-
-
-
Intrasellar: CSF fistula and pneumocephalus may occur. Rarely, vascular injury and hematoma may occur. However, the most common postoperative problem is CSF leak. It has been reported to occur in 1-4%. Under normal circumstances, CSF leakage is not expected when the suprachiasmatic system is not opened. However, due to the large suprasellar component of the tumor, CSF leakage may occur while the tumor is evacuated. In this case, the damaged diaphragmatic sella is covered with a fascia graft taken from the abdominal wall or fascia lata. If the sella remains empty, it is filled with muscle tissue, the sella base is strengthened with a small septal bone, and the sella base is covered with fibrin adhesives. The patient is placed on lumbar drainage and drainage is continued for 2-5 days depending on the condition. Jho recommends the obliteration of the abdominal fat tissue and the sphenoid sinus after repair of the sella floor defect with bone grafting, and states that he encountered a CSF leak in a case in which he did not perform fat obliteration (35) (34) Additionally, reconstruction with a pedicled nasoseptal flap or nasal turbinate graft also prevents CSF fistula. Rarely, meningitis may develop due to CSF leak. (27) Therefore, it is important for such patients to be treated with antibiotics for a long time.
-
Pituitary Apoplexy
Pituitary apoplexy describes an acute clinical picture accompanied by headache, vomiting, vision loss, ophthalmoplagia and confusion. Small infarct areas and hemorrhages within the adenoma may be seen in imaging studies, surgery or histopathological examination, but since these are usually asymptomatic, they do not cause pituitary apoplexy.
The first case of catastrophic bleeding into a pituitary adenoma was reported by Pearce Bailey in 1898. (2) In 1950, Brougham et al. They defined the clinic of pituitary apoplexy in 5 cases by reviewing the previous literature.
Pituitary apoplexy can occur due to acute infarction or bleeding into an existing pituitary adenoma, and it is also used to describe bleeding into the pituitary gland, which contains a tumor (Figure 29). Apoplexy has been reported in pituitary adenomas as well as craniopharyngiomas and lymphocytic hypophysitis cases. (58) While pituitary apoplexy clinic can develop within hours, apoplexy clinic can appear up to 2 days. Its pathophysiology is unknown.
The incidence of asymptomatic hemorrhage and necrosis in pituitary adenomas has been reported to be 14-22%, but the incidence of clinically symptomatic pituitary apoplexy as described has been reported to range from 0.6-9.1% in surgically treated pituitary adenomas. (62) (57)
Although pituitary apoplexy is usually spontaneous, it has been associated with many clinical conditions and medications such as head trauma, arterial hypertension, temporary increase in intracranial pressure, diabetes mellitus, cardiac surgery, dynamic tests to investigate pituitary function, GnRh analogue use, anticoagulation, estrogens, bromocriptine and radiotherapy. (50). These factors can be summarized as follows: 1- Decrease in blood flow into the pituitary gland, 2- Acute increase in blood flow into the pituitary gland, 3- Stimulation of the pituitary gland, 4- Anticoagulation status of the patient. (58)
The pathology that causes pituitary apoplexy may be ischemia within the adenoma, hemorrhage following ischemia, or just hemorrhage. However, the pathophysiology that causes infarction, hemorrhage, or a combination of these is not fully known. If the growth rate of the pituitary adenoma is faster than the development rate of the blood vessels, it may cause infarction and subsequent homorrhagia; As the tumor grows, the superior hypophyseal vessels may become compressed between the tumor and the diaphragm sella, causing ischemia, infarction and subsequent hemorrhage, or as suggested by some authors, hemorrhage may occur due to intrinsic vasculopathy of the vessels within the pituitary adenoma. When hemorrhage occurs, it is usually not possible to understand whether ischemia first occurred or whether hemorrhage occurred directly. (57) If the pathophysiology that causes pituitary apoplexy is ischemia only, the clinical picture of apoplexy is less severe than hemorrhage following ischemia or hemorrhage alone. The pressure within the tumor probably increases with infarction, but this increase is not as much as in hemorrhage. When there is hemorrhage, the neural structures around the adenoma, the optic chiasm and both cavernous sinuses are under more pressure. At the University of Virginia, Edward R. Laws et al. In their series of 62 cases, it was reported that the neurological examination was normal in 77% of the cases with only infarction within the adenoma, and that the neurological examination was normal in only 37.8% of the cases with hemorrhage. (57)
Pituitary apoplexy is typically seen in pituitary macroadenomas, but the adenoma subtype with a higher risk of apoplexy has not been reported.
Pituitary apoplexy is mostly seen in patients previously unknown to have pituitary adenoma. At the University of Virginia, Edward R. Laws et al. This rate was reported as 81% in their series of 62 cases. (58)
The first step in the treatment of a patient presenting with pituitary apoplexy is close monitoring and, if necessary, corticosteroid administration. Early surgery is controversial, especially if there is no progressive neuroophthalmological loss. If there is vision loss, immediate decompression is recommended. (50) However, pituitary apoplexy is a life-threatening clinical condition. The emergence and clinical course of apoplexy can range from a relatively benign disease to major neurological deficits and even death. Edward R. Laws et al. at the University of Virginia. In their series of 62 cases, minor neurological deficits were reported as 35.1%, major neurological deficits as 16.2%, and the death rate as 8%. (57)
Transsphemoidal surgery, craniotomy or conservative approach can be applied in treatment. In the series of Edward Laws et al., transsphenoidal surgery was applied in 77% of the patients, craniotomy in 16%, and conservative treatment in 5% of the patients, respectively. One patient died before treatment. It has been reported that the author used craniotomy mostly in his first cases. (58)
Visual acuity, visual field, and cranial nerve functions after apoplexy treatment were examined, and pre- and post-treatment records of 55 patients were found in Edward Laws' series. Accordingly, visual acuity was normal in 69% of the patients, visual acuity improved and normalized in 16%, but there was no change in 4%. The visual field examination of 52 patients could be compared and according to this, the visual field was normal in 73% of the patients, and improved but could not return to normal in 21%. It was found to be unchanged in 4%, and worsened in 2%. Cranial nerve deficits could be compared in 54 patients, and it was reported that 80% of the patients were normal, 20% improved but could not return to normal. No patient was reported with no change or worsening in cranial nerve examination at the end of follow-up. Endocrine replacement therapy was applied to 83% of the patients. Temporary diabetes insipidus was observed in 5% of the patients and permanent diabetes insipidus was observed in 9%. According to the results of the same series, 60% of the patients recovered without symptoms, 19% recovered with acceptable symptoms, 6% recovered with disabling symptoms, 5% died due to apoplexy, and 10% died for reasons other than apoplexy.
As a result, three main elements are recommended in the treatment of pituitary apoplexy.
1-High dose corticosteroid treatment
2- Careful treatment of fluid electrolyte balance
3-Performing transsphenoidal decompression. Although urgent decompression is controversial, it is especially recommended in patients with progressive neurological deficits. Motta et al. They reported that the mortality rate was higher in cases that were not treated surgically. (14)
Randeva et al. If transsphenoidal surgery cannot be performed within the first 8 days (73%), it should be performed laterThey reported that it was more effective in neuro-ophthalmological recovery compared to (42%) and they especially emphasized the use of transphenodal surgery in the treatment of apoplexy due to its lower risk. (50)
As a result, pituitary apoplexy is an important clinical condition that is life-threatening, requires urgent treatment, and must be treated with neurosurgical intervention, especially if progressive neurological deficits develop. As explained in the previous sections of the article, endoscopic transsphenoidal surgery is a recommended treatment for patients with pituitary apoplexy because it has a relatively low risk, provides better imaging and allows wider decompression.

Picture 29: Pituitary adenoma causing apoplexy
Conclusion
Endoscopic pituitary surgery;
• preservation of paranasal sinus anatomy and physiology,
• providing better images,
• safer approach,
• fewer complications
For these reasons, it is increasingly finding a place in neurosurgery practice. Today, in addition to sellar-parasellar pathologies, many skull base tumors and even vascular pathologies can be treated with endoscopic methods. However, it should not be forgotten that endoscopic pituitary surgery is a team and technology job. The navigation system is an important guide. Endoscopic pituitary surgery should not be attempted by teams that do not have sufficient endoscopic equipment and experience.
resources
1. Ahn JY, Jung JY, Kim J, Lee KS, and Kim SH. How to overcome the limitations to determine the resection margin of pituitary tumors with low-field intra-operative MRI during trans-sphenoidal surgery: usefulness of Gadolinium-soaked cotton pledgets. Acta Neurochir (Wien) 150: 763-771; discussion 771, 2008.
2. Bailey P. Pathological report of a case of acromegaly, with
special reference to the lesions in the hypophysis cerebri and in the
thyroid gland; and a case of haemorrhage into the pituitary.
. Philadelphia Medical Journal 789-792., 1898.
3. Berker M. Endoscopic pituitary surgery. Turkish Journal of Neurosurgery 89-92, 2006.
4. Bushe KA, and Halves E. [Modified technique in transsphenoidal operations of pituitary adenomas. Technical note (author's transl)]. Acta Neurochir (Wien) 41: 163-175, 1978.
5. Cappabianca P, Alfieri A, Thermes S, Buonamassa S, and de Divitiis E. Instruments for endoscopic endonasal transsphenoidal surgery. Neurosurgery 45: 392-395; discussion 395-396, 1999.
6. Cappabianca P, Cavallo LM, Colao A, and de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 97: 293-298, 2002.
7. Carrau RL, Jho HD, and Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope 106: 914-918, 1996.
8. Carrau RL, Kassam AB, and Snyderman CH. Pituitary surgery. Otolaryngol Clin North Am 34: 1143-1155, ix, 2001.
9. Ceylan S, Koc K, and Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg 112: 99 -107, 2010.
10. Choe JH, Lee KS, Jeun SS, Cho JH, and Hong YK. Endocrine outcome of endoscopic endonasal transsphenoidal surgery in functioning pituitary adenomas. J Korean Neurosurg Soc 44: 151-155, 2008.
11. Cooke RS, and Jones RA. Experience with the direct transnasal transsphenoidal approach to the pituitary fossa. Br J Neurosurg 8: 193-196, 1994.
12. Cushing H. Surgical experiences with pituitary disorders. JAMA 1515-1525, 1914.
13. D'Haens J, Van Rompaey K, Stadnik T, Haentjens P, Poppe K, and Velkeniers B. Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas: a retrospective comparison with traditional transsphenoidal microsurgery in the same institution. Surg Neurol 72: 336-340, 2009.
14. da Motta LA, de Mello PA, de Lacerda CM, Neto AP, da Motta LD, and Filho MF. Pituitary apoplexy. Clinical course, endocrine evaluations and treatment analysis. J Neurosurg Sci 43: 25-36, 1999.
15. de Divitiis E, Cappabianca P, and Cavallo LM. Endoscopic transsphenoidal approach: adaptability of the procedure to different seller lesions. Neurosurgery 51: 699-705; discussion 705-697, 2002.
16. Duz B, Harman F, Secer HI, Bolu E, and Gonul E. Transsphenoidal approaches to the pituitary: a progression in experience in a single centre. Acta Neurochir (Wien)150: 1133-1138; discussion 1138-1139, 2008.
17. El-Banhawy OA, Halaka AN, Altuwaijri MA, Ayad H, and El-Sharnoby MM. Long-term outcome of endonasal endoscopic skull base reconstruction with nasal turbinate graft. Skull Base 18: 297-308, 2008.
18. Ergin NB, M. Altınörs, N. Dal, T. The place of endoscopy in transnasal pituitary surgery. . Turk Arch ORL 36: 50-54, 1998.
19. Ergin NB, M. Altınörs, N. Dal, T. The place of the endoscope in transnasal pituitary surgery. . K Journal of BB and Head and Neck Surgery 7: 67-70, 1999.
20. Eskandari R, Amini A, Yonemura KS, and Couldwell WT. The use of the Olympus EndoArm for spinal and skull-based transsphenoidal neurosurgery. Minim Invasive Neurosurg 51: 370-372, 2008.
21. Evliyaoğlu Çİ, K. Keskin, G. Ceylan, S. Endoscopic endonasal transsphenoidal pituitary surgery. Turkish Journal of Neurosurgery 93-99, 2001.
22. Gamea A, Fathi M, and el-Guindy A. The use of the rigid endoscope in trans-sphenoidal pituitary surgery. J Laryngol Otol 108: 19 -22, 1994.
23. Gardner PA, Kassam AB, Rothfus WE, Snyderman CH, and Carrau RL. Preoperative and intraoperative imaging for endoscopic endonasal approaches to the skull base. Otolaryngol Clin North Am 41: 215-230, vii, 2008.
24. Grant JA. Victor Darwin Lespinasse: a biographical sketch. Neurosurgery 39: 1232-1233, 1996.
25. Griffith HB, and Veerapen R. A direct transnasal approach to the sphenoid sinus. Technical note. J Neurosurg 66: 140-142, 1987.
26. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, and Mintz A. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116: 1882-1886, 2006.
27. Han ZL, He DS, Mao ZG, and Wang HJ. Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary macroadenoma surgery: experience from 592 patients.Clin Neurol Neurosurg 110: 570-579, 2008.
28. Hardy J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg 185-217, 1969.
29. Haruna S, Otori N, Moriyama H, and Kamio M. Endoscopic transnasal transethmosphenoidal approach for pituitary tumors: assessment of technique and postoperative findings of nasal and paranasal cavities. . ;Auris Nasus Larynx 34: 57-63, 2007.
30. Heo KW, and Park SK. Rhinologic outcomes of concurrent operation for pituitary adenoma and chronic rhinosinusitis: an early experience. Am J Rhinol 22: 533-536, 2008.
31. Hirsch O. Endonasal method of removal of hypophyseal tumors. With a report of two successful cases. . JAMA 772-774, 1910.
32. Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, and Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope 102: 198-202, 1992.
33. Jho HD, and Jho D. H. editor. Schmidek & Sweet Operative Neurosurgical Techniques indications, methods and results “Endoscopic transsphenoidal surgery”. Philadelphia PA: Saunders Elsevier, 2006, p. 332-347.
34. Jho HD, and Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg 87: 44-51, 1997.
35. Jho HD, Carrau RL, Ko Y, and Daly MA. Endoscopic pituitary surgery: an early experience. Surg Neurol 47: 213-222; discussion 222-213, 1997.
36. Kabil ME, JB Shahinian, HK. Fully endoscopic transnasal versus transseptal transphenoidal pituitary surgery. . Neurosurg Q 190-196, 2005.
37. Kassam A, Snyderman CH, Mintz A, Gardner P, and Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 19: E3, 2005.
38. Kassam A, Snyderman CH, Mintz A, Gardner P, and Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 19: E4, 2005.
39. Kassam AB, Gardner P, Snyderman C, Mintz A, and Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 19: E6, 2005.
40. Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, Zanation A, Duz B, Stefko ST, Byers K, and Horowitz MB. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg 114: 1544-1568, 2011.
41. Koren I, Hadar T, Rappaport ZH, and Yaniv E. Endoscopic transnasal transsphenoidal microsurgery versus the sublabial approach for the treatment of pituitary tumors: endonasal complications. Laryngoscope ;109: 1838-1840, 1999.
42. Levy ML, Nguyen A, Aryan H, Jandial R, Meltzer HS, and Apuzzo ML. Robotic virtual endoscopy: development of a multidirectional rigid endoscope. Neurosurgery62 Suppl 2: 599-606, 2008.
43. Maroon JC. Skull base surgery: past, present, and future trends. Neurosurg Focus 19: E1, 2005.
44. Minet WW, Sommer DD, Yousuf K, Midia M, Farrokhyar F, and Reddy K. Retrospective comparison of an endoscopic assisted versus a purely endoscopic approach to sellar tumor resection . J Otolaryngol Head Neck Surg 37: 759-767, 2008.
45. O’Malley BW, Jr., Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, Bohman LE, and Leibowitz JM. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus 25: E10, 2008.
46. Oertel J, Gen M, Krauss JK, Zumkeller M, and Gaab MR. The use of waterjet dissection in endoscopic neurosurgery. Technical note. J Neurosurg 105: 928-931, 2006.
47. Oertel J, Krauss JK, and Gaab MR. Ultrasonic aspiration in neuroendoscopy: first results with a new tool. J Neurosurg 109: 908-911, 2008.
48. Prevedello DM, Pinheiro-Neto CD, Fernandez-Miranda JC, Carrau RL, Snyderman CH, Gardner PA, and Kassam AB. Vidian nerve transposition for endoscopic endonasal middle fossa approaches. Neurosurgery 67: 478-484, 2010.
49. Raappana A, Koivukangas J, and Pirila T. 3D modeling-based surgical planning in transsphenoidal pituitary surgery–preliminary results. Acta Otolaryngol 128: 1011-1018 , 2008.
50. Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, and Wass JA. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf)51: 181-188, 1999.
51. Reuter M. [Philipp Bozzini (1773-1809): The endoscopic idealist]. Urologe A 45: 1084-1088, 1090-1081, 2006.
52. Rodziewicz GS, Kelley RT, Kellman RM, and Smith MV. Transnasal endoscopic surgery of the pituitary gland: technical note. Neurosurgery 39: 189-192; discussion 192-183, 1996.
53. Rosen MR, Saigal K, Evans J, and Keane WM. A review of the endoscopic approach to the pituitary through the sphenoid sinus. Curr Opin Otolaryngol Head Neck Surg 14: 6-13, 2006.
54. Roth J, Singh A, Nyquist G, Fraser JF, Bernardo A, Anand VK, and Schwartz TH. Three-dimensional and 2-dimensional endoscopic exposure of midline cranial base targets using expanded endonasal and transcranial approaches. Neurosurgery 65: 1116-1128; discussion 1128-1130, 2009.
55. Schaberg MR, Anand VK, Schwartz TH, and Cobb W. Microscopic versus endoscopic transnasal pituitary surgery. Curr Opin Otolaryngol Head Neck Surg 18: 8-14 , 2010.
56. Schwartz TH, Fraser JF, Brown S, Tabaee A, Kacker A, and Anand VK. Endoscopic cranial base surgery: classification of operative approaches. Neurosurgery 62: 991-1002; discussion 1002-1005, 2008.
57. Semple PL, De Villiers JC, Bowen RM, Lopes MB, and Laws ER, Jr.Pituitary apoplexy: do histological features influence the clinical presentation and outcome? J Neurosurg 104: 931-937, 2006.
58. Semple PL, Webb MK, de Villiers JC, and Laws ER, Jr. Pituitary apoplexy. Neurosurgery 56: 65-72; discussion 72-63, 2005.
59. Shah RN, Surowitz JB, Patel MR, Huang BY, Snyderman CH, Carrau RL, Kassam AB, Germanwala AV, and Zanation AM. Endoscopic pedicled nasoseptal flap reconstruction for pediatric skull base defects. Laryngoscope 119: 1067-1075, 2009.
60. Unlu A, Meco C, Ugur HC, Comert A, Ozdemir M, and Elhan A. Endoscopic anatomy of sphenoid sinus for pituitary surgery. Clin Anat 21 : 627-632, 2008.
61. Wagenmakers MA, Netea-Maier RT, van Lindert EJ, Timmers HJ, Grotenhuis JA, and Hermus AR. Repeated transsphenoidal pituitary surgery (TS) via the endoscopic technique: a good therapeutic option for recurrent or persistent Cushing's disease (CD). Clin Endocrinol (Oxf) 70: 274-280, 2009.
62. Wakai S, Fukushina, T., Teramoto, A. & Sano, K. . Pituitary apoplexy: its incidence and clinical significance. J Neurosurg 187-193., 1981.
63. Yano S, Kawano T, Kudo M, Makino K, Nakamura H, Kai Y, Morioka M, and Kuratsu J. Endoscopic endonasal transsphenoidal approach through the bilateral nostrils for pituitary adenomas. Neurol Med Chir (Tokyo) 49: 1-7, 2009.
64. Yue JX, Zhang S, Kong WJ, Wang YJ, Xiong XG, and Zhu LX. Trans-superior meatus endoscopic surgery of sphenoidal sinus and sellar area: a surgical technique for lesion of sellar area. Acta Otolaryngol 128: 1233-1237, 2008.
65. Zhang Y, Wang Z, Liu Y, Zong X, Song M, Pei A, Zhao P, Zhang P, and Piao M. Endoscopic transsphenoidal treatment of pituitary adenomas . Neurol Res 30: 581-586, 2008.